About NABL:
NABL is a registered society under the Societies Registration Act 1860. It operates as an autonomous body under the aegis of in the Department of Science and Technology (DST), Ministry of Science and Technology, Government of India. NABL has been established with the objective of providing Government, Industry Associations and Industry in general with a scheme of Conformity Assessment Body’s accreditation which involves third-party assessment of the technical competence of testing including medical and calibration laboratories, proficiency testing provides and reference material producers. The laboratory accreditation services to testing and calibration laboratories are provided in accordance with ISO/ IEC 17025: 2005 “General Requirements for the Competence of Testing and Calibration Laboratories” and ISO 15189: 2012 “Medical laboratories — Requirements for quality and competence”. The accreditation to Proficiency testing providers are based on ISO/IEC 17043 :2010 “Conformity assessment — General requirements for proficiency testing” and to reference material producers based on ISO Guide 34:2009 – General requirements for the competence of reference material producers ”The fields, disciplines and groups for which the accreditation services are offered are listed in “Scope of NABL Accreditation”. NABL offers accreditation services in a non-discriminatory manner. These services are accessible to all testing including medical and calibration laboratories, proficiency testing providers and reference material producers in India and other countries in the region, regardless of the size of the applicant CAB or its membership of any association or group or number of CABs already accredited by NABL. NABL has established its accreditation system in accordance with ISO/ IEC 17011: 2004 „Conformity Assessment – General requirements for Accreditation bodies accrediting conformity assessment bodies”. NABL accreditation system also takes the note of the requirements of Mutual Recognition Arrangements (MRAs) of which NABL is a member. NABL publishes documents for the CABs, Assessors and its own use. A list of NABL documents is given at the end of this document. All NABL documents meant for the use by persons outside NABL, are available on NABL website www.nabl-india.org, free of cost.
Benefits of Accreditation:
Formal recognition of competence of a laboratory by an Accreditation body in accordance with international criteria has many advantages:
- Increased confidence in Testing/ Calibration Reports issued by the laboratory.
- Better control of laboratory operations and feedback to laboratories as to whether they have sound Quality Assurance System and are technically competent.
- Potential increase in business due to enhanced customer confidence and satisfaction.
- Customers can search and identify the laboratories accredited by NABL for their specific requirements from the NABL Web -site or Directory of Accredited Laboratories.
- Users of accredited laboratories enjoy greater access for their products, in both domestic and international markets.
- Savings in terms of time and money due to reduction or elimination of the need for retesting of products.
The benefits of proficiency testing are widely recognized. These include:
- Comparison of a facility’s performance with that of other participating (peer) facilities
- Monitoring of a long-term facility performance
- Improvement in the performance of tests/calibrations following investigation and identification of the cause(s) of unsatisfactory PT performance, and the introduction of corrective action to prevent re- occurrence
- Staff education, training and competence monitoring
- Evaluation of methods, including the establishment of method precision and accuracy
- Estimation of measurement uncertainty
- Contribution to the facility’s overall risk management system
- Confidence building with interested parties, e.g. customers, accreditation bodies, regulators, specifiers.
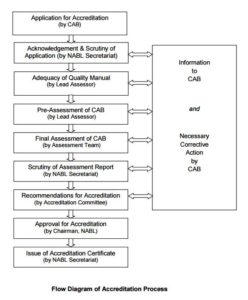
Accreditation Procedure
OUR CONSULTANCY SCOPE OF SERVICE:
- Study existing Laboratory infrastructure and recommendation of changes requirement accordingly. We conduct total GAP analysis as per the requirement of Accreditation body specific criteria such as NABL 103, NABL 107, NABL 102, NABL 104 etc for Industrial testing parameter and for medical laboratories we follow NABL 112
- Training on standard requirement to Quality Manager including Quality control requirement
- Conducting periodical 4 days Training
- Conducting quality awareness Training program for laboratory employees as per Accreditation requirement
- To Assist Quality Manager in development of Laboratory Quality Management System
- 4 level Quality Management system document preparation which include preparation of Quality manual, Management system procedure preparation and deliver for guideline on preparation of standard operating procedures for test
- Assisting Calibration services, procurement of CRM
- Assisting in development of secondary reference and control materials
- Assisting in participation of inter laboratory comparison or Proficiency testing for Industrial testing and EQAS programme for Medical testing laboratories
- Development of all formats required to establish quality management system
- Assisting you preparation of internal audit and preparation of Management review meeting minutes
- Assisting you closure of Non-conformance, assisting you closure of all kind of Complaint lodged against the laboratory.
- To deliver you all sorts of guidance till the completion of accreditation process